Can you imagine powering an electric car with candles? New research shows that soot from burning candles could be the perfect anode material for larger batteries.
How do Lithium Ion Batteries work?
Lithium ion batteries use negatively charged lithium ions which travel to a positive electrode while discharging. When the battery is charged the lithium ions are put back onto the negative electrode allowing an endless cycle of powering electronics, and charging from another source.
If lithium ions are the cathode then what is the anode? Researchers have been long looking for the best candidate.
Candle Soot Lithium Ion Batteries.
Graphite is considered the highest grade of coal and the most stable form of carbon in the real world. Graphite is nearly one dimensional, with atoms arranged like a honeycomb. Graphite is shockingly easy to make too. Take a pencil, rub it on paper and apply a piece of scotch tape. Carefully remove the tape and voila! Graphite. Graphite has shown to be effective in small lithium ion batteries. On a larger scale, however, the structure of carbon can’t create a large enough current to be useful.
Another form of carbon, equally easy to make, however, is showing a lot of promise. Candle soot. When you burn a candle tiny carbon nanoparticles are released. This easy to make candle soot has already been showing promise int he areas of super hydrophobic coatings. Some experiments are even testing candle soot in solar cells!
Just recently a team of researchers investigated how candle soot would work as a fractal-like carbon nanoparticle network in lithium ion batteries.
How do you make carbon nanoparticles?
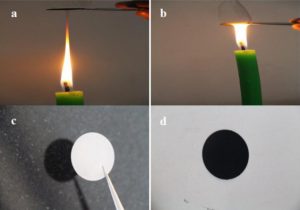
You just put a piece of metal at the tip of a candle flame.
Wait for a little bit and voila! The capacities of carbon nanoparticles derived from the soot had almost a 25% capacity retention after 100 cycles! Not only that, it showed a 91% efficiency, making candle soot the perfect candidate for high rate lithium ion batteries!
Kids science – cool experiments to go deeper.
Explore the difference between graphite, graphene and carbon nanoparticles.
This is the perfect kids science activity to explore the difference between graphite, graphene and carbon nanoparticles. Best of all, this cool science experiment takes hardly any supplies. You just need a pencil, scotch tape, a candle and a piece of metal.
Take your pencil and rub it on a piece of paper. Then use your piece of scotch tape to gently place it ontop of the pencil marking. When you remove it you have a layer of graphite!
To make carbon nanoparticles you can place the piece of metal at the tip of the candle as it is burning. Be careful, because this can and will get hot! Use tweezers, tongs or another device to hold it safely! If you let the candle burn for a minute or two you will start to notice black buildup on the bottom of the metal. That is your carbon nanoparticles.
Of course, plain graphite is pencil lead. Now you can look at all three side by side. You essentially ave 1D carbon (nanoparticles), 2D carbon (graphene), and 3D carbon (graphite)!