Purple cabbage juice is a popular natural pH indicator that is used in chemistry labs to show how the conformation of a molecule can change the colors we see. In this natural pH indicator lab, you will use purple cabbage juice to determine the pH of various household items. Some chemicals you can test the pH of are vinegar, baking soda, shampoo, juices, and more!
Scroll down to learn about how a molecule called anthocyanin is the culprit for the color changes, and how the anthocyanin pH indicator works.
How to make purple cabbage juice for pH indicator experiments.

Now gather up a ton of household items: salt, sugar
, milk, vinegar, baking soda
… Your imagination is the limit!
You will need our fun kids science activity download. Our cabbage natural pH indicator lab is a great activity for all ages. Along the way, you will get to read Rosie’s story, figure out what she really added to her drink and solve some acid/base puzzles. There is also a cabbage indicator color chart in the lab so you know what to expect and can use the colors you see to convert into a pH.

Thrive Leads Shortcode could not be rendered, please check it in Thrive Leads Section!
What gives red or purple cabbage it’s color?
Red cabbage juice is purple because of anthocyanin, a pigment often found in flowers, fruits, and leaves. In fact, it is the cause of many of the reds, blues, purples, and oranges we see around us everywhere.
How does cabbage juice work as a pH indicator?
 Cabbage juice itself isn’t the reason the color changes as a function of the pH. Anthocyanin, a molecule found in natural pH indicators is the reason for the color change.
Cabbage juice itself isn’t the reason the color changes as a function of the pH. Anthocyanin, a molecule found in natural pH indicators is the reason for the color change.
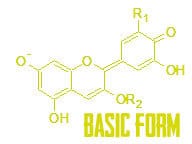
Colors of the world around us are determined by how molecules like anthocyanin absorb light. They can absorb light into their outer shell electrons (called valence electrons). The energy absorbed into the bonds through light is light that will never reach our eyes, so if red light is absorbed, a substance would look blue.
When we add in acid we change the structure of anthocyanin. That is because adding acid is adding in a bunch of hydrogen ions (H+), and oxygen can bind with that hydrogen. Can you see the difference between the red, acidic, formation of anthocyanin and the purple, neutral, formation of anthocyanin?
Adding base is like adding a bunch of hydroxide ions (OH-). This also changes the molecule. Can you spot the difference?
All of these changes to the molecule changes the bonds. With different bonds, we absorb different energies. That means absorbing different wavelengths, or colors, of light! Think about it this way. When you are tuning a guitar tighter you hear a higher note. When you tune it lower you hear a lower note. Those different frequencies correspond to different wavelengths. In light the wavelength determines the color. Tuning the bonds in the molecules changes the color absorbed!
Extending your cabbage pH indicator lab with more activities.
Flavinoids, Flavinoids, Everywhere.
The molecule anthocyanin, a flavonoid, is found in the coloring of fruits, vegetables and even leaves all around us. Blackberries, blueberries, cherries, plums, red apples, wines and even red beets all are chalk full of flavonoids. Experiment with other plants that could be used as a pH indicator like cabbage. Which one works the best? You can see our review of beets as a pH incidator.
Purple cabbage as a pH indicator to write spy messages.
Red cabbage juice will only change color where a strong acid or strong base is present. Using full-strength lemon juice write a secret message on a piece of paper. After it dries use red cabbage juice to paint over the paper. What do you see?
How to make homemade pH paper.
You can make your own pH paper, also known as litmus paper, using red cabbage juice and coffee filters. Just soak the filters, dry, and cut into strips. Now you can take your pH testing out of the lab and into the wild!


10 thoughts on “Color changing cabbage as a natural pH indicator”
Comments are closed.